 SPERM DILUTION
SPERM DILUTION | CONTENTS | OVERHEADS | GLOSSARY | REFERENCES | SKILLS | CLASSIC |
 SPERM DILUTION
SPERM DILUTION 10/8/97
|
SUMMARY: This is the last lesson in the DILUTION series and probably the most difficult. Students are asked to make a sperm dilution series and calculate the concentration of sperm in each dilution. Students will do the following: 1. make serial dilutions |
|
| TIMING | BACKGROUND | MATERIALS | PROCEDURE | MATH | IMPLICATIONS | EVALUATION |
(see SIMPLE DILUTION and SIMPLE DILUTION 2 lesson plans)
Being able to do a serial dilution of sperm concentration is essential to being able to do experiments in fertilization and development with sea urchins.
For some reason the concept of a serial dilution is EXTREMELY hard for students to grasp. (even at the undergraduate and graduate levels). It is a very powerful way of determining the ideal concentration over a large range of values. Doing a simple linear dilution from 0-10 in steps of 2 is straight forward and easy. What do you do when the values are from 1:1000 to 1:100,000 and linear "steps" will not give valid answers. The solution is to use a serial dilution. The difference between the two methods is the difference between addition and multiplication.
Simplest case:
A sample is diluted 1:1 with a solvent (sea water in the case of sea urchin sperm). The result is now half the concentration of the original. If we now do a further 1:1 dilution of the dilution we have 1/2 times 1/2 or 1/4 the original concentration. Continuing the series we would end up with the following:
|
Tube # |
% initial |
fraction of original |
|
1 |
100 |
1 |
|
2 |
50 |
1/2 |
|
3 |
25 |
1/4 |
|
4 |
12.5 |
1/8 |
|
5 |
6.25 |
1/16 |
|
6 |
3.125 |
1/32 |
In each case we multiplied the one above by 1/2 to get the next concentration in the series. We could just as easily have used a factor of say 10, whereby we would multiply each succeeding concentration by 1/10.
|
[In fact this can be seen easily by using the spectrophotometer from the Simple Dilution lesson and substituting a GREEN filter for the BLUE one and using a 1% solution of phenolphthalein in a basic solution for the "dye" (in place of the Coca-Cola). The magenta color of the dye is very intense and even diluting from the initial concentration of 1 part per 100 (1%) to a final concentration of 1 part per million (0.0001%) a reading can still be made. Using a LOG-LOG graph paper to record the results one can figure out an unknown over a quite large range of concentrations.] |
A serial concentration is useful in determining the "ideal" sperm concentration to fertilize a particular batch of eggs. The sperm may be under conditions were they are not so good or the eggs themselves may have been affected by a particular treatment or exposure to say an environmental toxin. Sperm density directly from the male sea urchin will vary depending on the health of the animal, season and how much sea water mixed with the sperm during collection. So an accurate way of determining concentration would also be useful. By correlating sperm concentration with a reading from our homemade spectrophotometer we can do a very quick check of sperm from a new source.
EXAMPLE TABLE: NOTE: readings on the "spectrophotometer" will vary considerably, depending on your setup.
|
Tube # |
Dilution |
# of sperm per small square |
K-Ohms (spectro-photometer) |
sperm per ml (calculated) |
|
1 (stock) |
1:1000 |
~4 |
1310 |
16,000,000 |
|
2 |
1:2000 |
~2 |
1120 |
8,000,000 |
|
3 |
1:4000 |
~1 |
1030 |
4,000,000 |
|
4 |
1:8000 |
~0.5 |
1000 |
2,000,000 |
|
5 |
1:16,000 |
Too dilute |
970 |
1,000,000 |
|
6 |
1:32,000 |
Too dilute |
950 |
500,000 |
|
7 |
1:64,000 |
Too dilute |
940 |
250,000 |
|
8 |
1:128,000 |
Too dilute |
960 |
125,000 |
|
BLANK |
-------- |
0 |
960 |
0 |
USING A HEMACYTOMETER:
|
|
(photo courtesy of the online Fisher catalog) Place a small drop of suspension to be measured on one of the two raised platforms of the hemacytometer. Add the cover slip. |
|
|
(view through the hemacytometer) In the center of the field you will see the smaller set of squares. These are the ones you want to use. Recommend using the 40X objective to be able to count the number of sperm in the field. |
HEMACYTOMETER:
Each small square is 1/400 square millimeter (0.05 mm x 0.05 mm OR 50 microns x 50 microns). The depth is 0.1 mm. Thus the volume is 0.05 X 0.05 X 0.1 mm = 0.00025 cubic mm
1 cubic mm = 1 microliter = 1/1000 of a ml = 1/1,000,000 of a liter
SO, 0.00025 microliters is 0.00000025 ml or 1 four millionth of a milliliter. If we take the reading we found for the number of sperm in each tube and multiply it by 4,000,000 we will get the number of sperm per milliliter!
OK -- the sperm were too dense in the higher concentrations to see them and they were too dilute to see them in the lower concentrations. BUT, by using the math for making the dilutions BACKWARDS we should be able to fill in the rest of the table.
SPECTROPHOTOMETER:
The sperm were too dilute in the lower concentrations to read above pure sea water, but you should be able to see them at the higher ones and draw up a graph of the results. Plot the SPERM/ML against the K-OHMS reading
EXAMPLE:
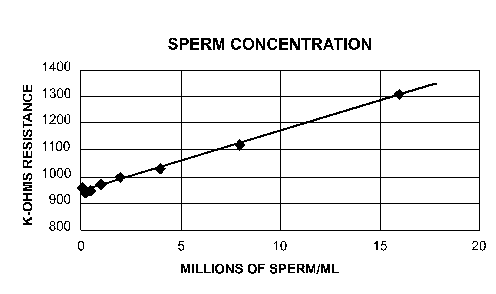 NOTE: The highest concentration is 16 million sperm per milliliter, and remember, this is still a 1:1000 dilution. That means that the sperm coming out of the sea urchin are at 16 billion sperm per milliliter!
NOTE: The highest concentration is 16 million sperm per milliliter, and remember, this is still a 1:1000 dilution. That means that the sperm coming out of the sea urchin are at 16 billion sperm per milliliter!
From a practical standpoint, in the experiments that follow in the FERTILIZATION and EXPERIMENTS lessons you will be able to put a "real" number on the sperm concentration that you used in your experiments. Since this number can vary for each experimental condition this provides another means of quantifying what is going on. Example: it may take ten times as much sperm in a "toxic" condition to fertilize a batch of eggs.