![]()
By Amro Hamdoun
An ongoing study to describe the effects of contaminants on the embryonic development of embryos in the Nile Delta Estuary.
Background:
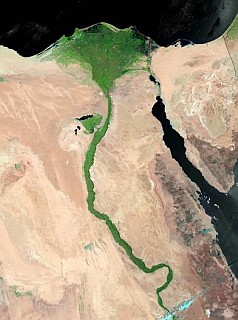 |
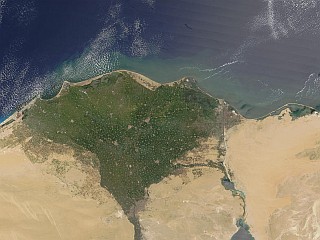
The Nile river forms the v-shaped delta in the north of Egypt . The Nile ’s contributes to rich agricultural productivity of the Delta and its outlets to the Mediterranean Sea are an important source of nutrients to the marine in environment. They are the major estuaries or “lakes” along the North African Coast. These estuaries are important feeding and nursery areas for many of the marine species in the region. Our project focuses on developing a bioassay system that could be used to detect the biological effects of anthropogenic contaminants, present in Nile effluents, on larval development. |
 |
 |
Fisheries:
 |
The slide shows traditional fisheries at Lake Manzalah the eastern Nile Delta estuary. |
 |
The Damietta branch of the Nile Delta in the Eastern Nile Delta is home to over 50% of Egypt ’s commercial fishing fleet. |
 |
Anthropogenic pollutants enter the Nile from a variety of sources including agricultural, industrial and municipal sources. |
Collecting and Spawning:
  |
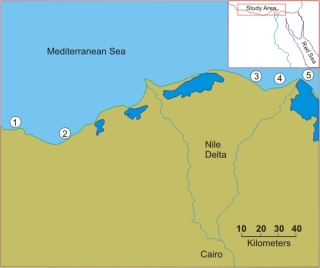
We used sea urchins because they are an excellent system for marine bioassays. At Al Mansourah University we characterized the urchins we collected from the clean sites. Transects of Study Sites 1 and 2. |
| Black sea urchins – Arbacia lixula were collected west of Marsaa Matrouh in the fall of 2004 for use in bioassays. |  |
 Dr. Mohammed Zyadah and Ms. Fatma El-Matary (M. Sc. Student) spawning urchins by injection of KCl. |
 Photo of Al Mansourah Univeristy, Damietta Branch. |
| A female urchin releasing dark purple eggs into seawater. |  |
 |
Immediately after addition of sperm fertilization is confirmed under the microscope. |
Development:
   |
|
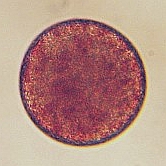
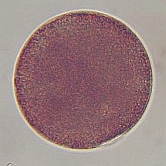
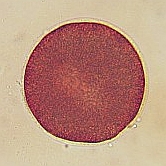
A. lixula egg (diameter is approximately 50 micrometers). Note the dark pigment granules which contribute to the color of the eggs.
Moments after fertilization the formation of a fertilization envelope can be seen at the point of sperm entry approximately at the 5:00 position. Within 20 minutes of fertilization the cell enters mitosis seen as elongation of the nucleus (light colored region in the center of the egg). |
|
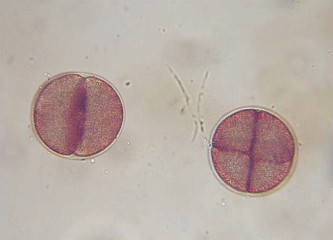 |
30-45 minutes after fertilization. First cleavage is completed and cleavages continue every 15 minutes at 22°C. |
   |
|
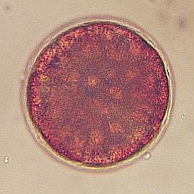
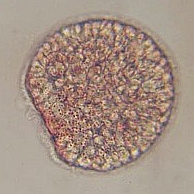
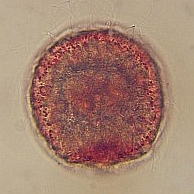
4-6hr after fertilization the embryo is forming an early multicellular blastula. 10hr post fertilization, the embryo has hatched from the envelope and is becoming an early gastrula. 16hr post fertilization the embryo gut is starting to form (gastrulation) and the swimming embryo has developed a large apical tuft of cilia. |
|
   |
|
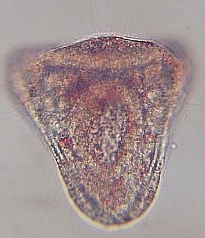
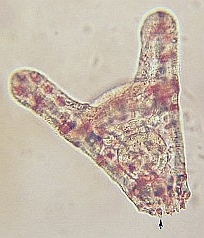
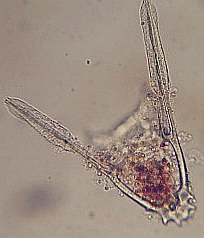
28 hr post fertilization the embryo is an early pluteus larva.
48 hr post fertilization the embryo has formed a full pluteus larva and the embryo is starting to form a skeletal “crown” at the base of larva. 72 hrs post fertilization, the larval skeleton known as spicules are elongating and the “crown” is fully formed. |
|
Experiments:
Exposing embryos to heavy metals present in Nile effluents, such as cadmium and copper, inhibits embryonic development.
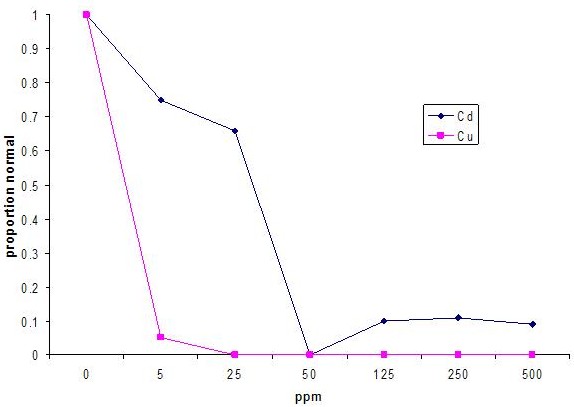
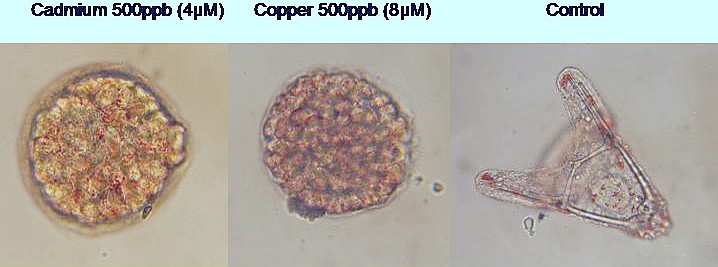
Cadmium and copper cause developmental delay or arrest in the early gastrula stages. Note that the cadmium exposed embryo is starting to exo-gastrulate.
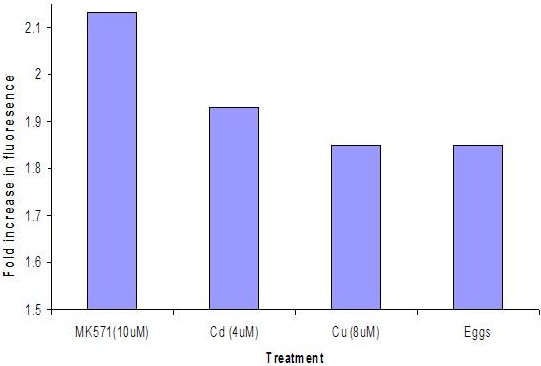
Efflux transporters are inhibited by the heavy metals. This inhibition is measured as increased accumulation of a fluorescent indicator dye inside the embryos.
Support:
This work was funded by the US-Egypt Joint Science Fund and the USDA.
This project is a collaboration between HMS, Stanford Unversity and Al-Mansoura University, Damietta.


Back to S.U.E Contents page.
Website by c. patton @ Hopkins Marine Station, Stanford University.