
Lauren Palumbi's Poster Presentation
3/23/2004
Lauren is a student at Robert Louis
Stevenson High School in Pebble Beach California.
This science fair project won numerous awards and will go onto the state level.
As such it
provides an excellent example of the level of work, we at Sea Urchin Embryology,
hope to
encourage and develop in all who use our site.
Note: Some resolution is lost going from a poster 3x4 feet in size to a web page.

|
Introduction
With the continual growth of the worlds’ population, many questions about the health of the environment have been raised. The concern for the pollution and it’s affects on environment and food sources is one of these issues. A standard test to asses the affects of a supposed contaminated water source is a sea urchin embryo bio-assay. The purpose of this experiment is using levels of recorded contaminates from the Monteray Bay area to ascertain it’s affect on the local ecosystem. The current amount of toxins detected in the runoff to the bay as documented in the “First Flush Report 2000” indicates that the runoff water is enough to affect sea urchin reproduction (1) However, once the runoff waters join with the ocean, the toxins become diluted and are therefore less hazardous. I predict that the measured amount of runoff toxins will affect the bio-assay, but when tested with the estimated dilution factor, not much of an affect will be noted until the higher concentrations of toxins. This issue is a critical one for the growing community because of the rising levels of runoff pollutants. Even with the dilution factor of the ocean, organisms near the shores will eventually be affected by the toxic levels of land runoff. My interest in this project stems from concern about the ocean’s future wellbeing in the Monteray Bay area as well as in other parts of the ocean. In experiments performed by
Dr’s G Pagano, JE Hose, and Dave Epel (though in slightly different
fashions) fertilization was tested as well as embryo development. Because
fertilization testing requires precision of the percent of success in
order to obtain readable results, it is simpler to gain results by using
fertilized embryos and observing early development. JE Hose’s tests
performed on embryos have measured growth within a 48 hour period. After
this time several tests were performed to ascertain the health of the
embryo. Because of the equipment needed to perform these tests I have
chosen to observe the time between each major cell division. The embryos
will perform mitosis 2-3 times and will be measured for the amount of
time it takes to do so. In the analysis of the runoff conducted by the Monterey Bay Sanctuary Citizen Watershed Monitoring Network levels of metals have been detected such as Copper, Nitrate, Zinc, Iron, and Lead. Other pollutants included Orthophosphate, E. coli bacteria, oil, grease, and dissolved and suspended solids. The substances chosen for this experiment were Copper, Lead, and Zinc. Using concentrations of Copper at 3.15 x 10(-7) molar, 3.15 x 10(-6) molar, and 3.15 x 10(-5) molar, Zinc at 3.82 x 10(-6) molar, 3.82 x 10(-5) molar, and 3.82 x 10(-4) molar, and Lead at 1.69 x 10(-7) molar, 1.69 x 10(-6) molar, and 1.69 x 10(-5) molar, will produce a wide range of possible effect. The chemical levels used in this experiment will be based on the levels recorded in the “First Flush Report” of 2000. However, because this is an annual event in the past few years more recent data has been collected. It was noted in the “First Flush Report” of 2002 that levels of Copper, Zinc, and Lead have increased at almost every test site. Affects of toxicity in the costal wildlife could already be noted. With the increase of these chemicals every year it can be assumed that the pollution levels will soon start to have an effect on more than the immediate costal regions, but could soon start to spread into the bay as well. |
|
Hypothesis
The healthy development of sea urchin embryos will be affected by the presence of toxic chemicals and pollutants. Depending on the concentration of the solution, mitosis of embryos will slow or cease all together. Lead, being the worst biohazard, should have more of an effect on the embryos than Zinc and Copper. |
|
Materials
13 test tubes |
|
Procedure
Mixing and diluting solutions: Mixing the solutions- The solutions used for this project were mixed using several different types of crystal salts. Zinc Chloride, Copper Chloride, and Lead Nitrate. To arrive at the correct dilutions we calculated values for grams per milliliter starting at about 10(-3) molar. Pouring 100 ml of distilled water into a glass container we then measured the mass of the crystals, and then mixed the proper amount of each into each of the containers. Diluting the solutions- After the solutions were mixed each had a concentration of 10(-3) to 10(-2) molar. This is about 100 times more concentrated than the highest desired solution concentration. To arrive at the correct value, one ml of solution was added to nine ml of sea water, making it a 1:10 concentration or 10(-4) molar. Using one ml the 10(-4) molar solution the procedure was repeated with another nine ml of sea water creating another 1:10 ratio or 10(-5) molar. This is the most toxic solution used on sea urchin gametes in this experiment. For further dilutions the procedure can be repeated. |
click on images and graphs for larger view
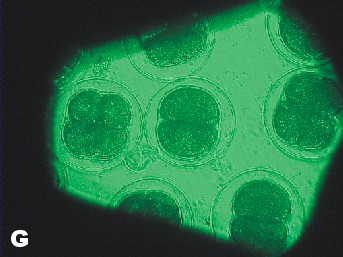 |
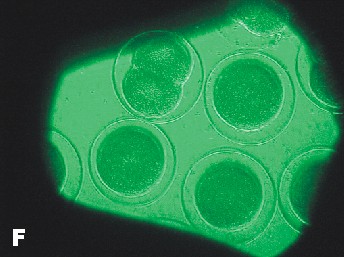 |
| Photo of Copper control at 5 ½ hours after fertilization. | Photo of lowest concentration of Zinc {10(-6)}. Taken approximately at the same time as the Copper control photo. |
|
Analysis
During the experiment it was observed that the control groups seemed to be dividing at a slightly faster rate than the affected samples. However, for the first few hours no change was readily apparent. Graph statistics show that the averages of the control had the highest amount of division after 4 ½ hours, but only by one percent. The first data was only taken for the control groups and the most concentrated solution of the three chemicals. One hour later all of the samples were measured. It was found that again the averages of the controls had a higher percent of division. However, the most concentrated solution of Zinc also had a high division percent. Seventeen hours after fertilization data was again taken for all of the samples, this time relating to the exact number of cells in each of the embryos. Lead samples had a much lower percentage of cells in the more advanced stages. Zinc’s results showed no conformity, generally having higher percentages of four cell embryos than the average of the controls. Copper however, proved to be definitely affected by the solutions, dropping in the number of 4 cells and 4+ cells and having an increase in abnormal cells. The last evaluation was 24 hours after fertilization. The Lead samples were the most distinguishably affected compared to the averaged controls. The percentage of 4 cells dropped while the number of abnormal cells increased. According to this data the rate of development between samples is drastically different. Even between controls the samples had radically different percentages of cell maturity. Of the three chemicals used to test toxicity, Lead and Copper were the ones to have the most drastic effect. Copper seems to create the most disruption in the health of the embryos. This proves that increasing levels of Copper and Lead in runoff water could potentially prove to effect sea urchin populations and therefore disrupt other species dependent on them. In disagreement with the Hypothesis Copper was proven to have the most drastic effect with increasing concentration. Lead did have an effect on development but to a less drastic degree than Copper. Embryos that were still at the single cell state after 24 hours can be considered to be halted in development. However, in none of the samples were the majority of the embryos single celled. Therefore, none of the solutions caused a total halt in embryo development. |
|
Conclusion The presence
of chemicals in the runoff waters of Monteray bay is a potential hazard
to the marine life and continued health of the bay. High levels of Copper,
Zinc, and Lead create at best a slow maturity rate of sea urchin embryos.
In the worst case the presence of such toxins can stop large numbers
of sea urchin embryos from ever reaching full maturity due to mutated
cells or a total cessation in development. The chemical that has the
greatest effect on sea urchin embryo development appears to be Copper.
The values used in this experiment were taken from the First Flush Report
of 2000. Since that time the amount of Copper in the water has risen
from 3.15 x 10(-7) molar to 4.7 x 10(-6) molar. In about another five
years the amount of copper could rise to 1.5 x 10(-5). That is roughly
953 ppb (parts per billion). It would take about 12 years at an annual
increase of 2.2 x 10(-6) molar of copper to reach the most concentrated
experimental copper value of 3.15 x 10(-5) or 2002 ppb. All of these
values greatly surpass the background concentration of copper in sea
water which is 3.1 x 10(-8) molar or 2 ppb. The next step in the process
of understanding our impact on the marine environment would be to see
how toxicity affected other organisms in the bay. Perhaps a main point
of interest for the public would be to see how the popular sea foods such
as shellfish, squid, and other fish are affected. Another possibility
would be to take a first hand look at the health of the populations of
sea urchins near the high points of runoff pollution. |
|
Bibliography
1. Dr. G. Pagano and others;
Cytogenetic, developmental, and biochemical effects of aluminum, iron,
and their mixture in sea urchins and mussels, Arch Environ Contam Toxicol.
1996 Nov;31(4):466-74. |
|
Acknowledgments
I would like to thank my Chemistry teacher Dr. Armando Galindo for pushing me to do this project. I would also like to thank Dr. Dave Epel for discussing methods of experimentation and the First Flush Report data. A large thank you to my father Dr. Stephen Palumbi for assisting me in collecting sea urchin gametes, taking photographs, and removing a sample for later observation. Thank you to Cathy Munsch and Dr. Dave Epel for providing me with samples of Zinc Chloride, Copper Chloride, and Lead Nitrate. Thank you to my cousin Jucinda for assistance in labeling and mixing dilutions of chemicals. A large thank you goes to Katherine Gibson, Chris Patton, and Dr. Julie Alipaz for helping with the layout for the poster board. And lastly, thank you to the Monterey Bay Sanctuary Citizen Watershed Monitoring Network for the public data of the First Flush Reports. |